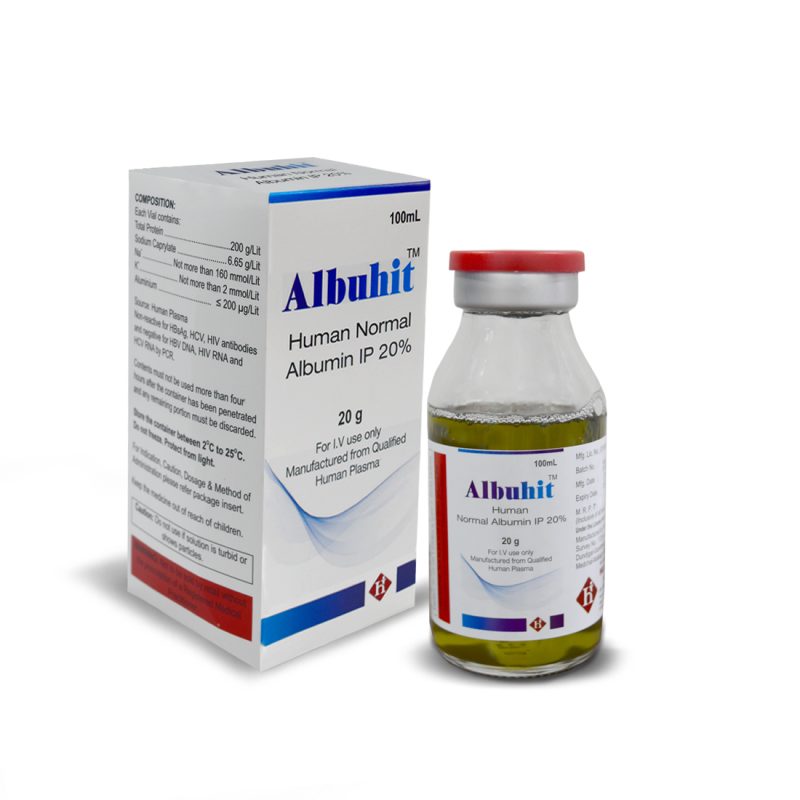
Albuhit
Human Normal Albumin IP 20%
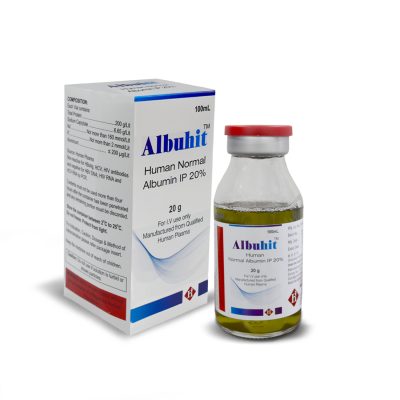
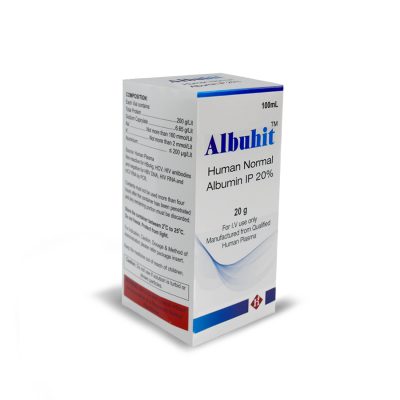
COMPOSITION:
- Each Vial contains:
- Total Protein ……………………….200 g/Lit
- Sodium Caprylate ……………… 6.65 g/Lit
- Na+……….. Not more than 160 mmol/Lit
- K+…………….. Not more than 2 mmol/Lit
- Aluminium …………………… ≤ 200 μg/Lit
For I.V. Use
2g/10mL, 4g/20mL, 10g/50mL & 20g/100mL
Manufactured from qualified Human Plasma
DESCRIPTION
Human Normal Albumin IP 20% solutions is a sterile, non-pyrogenic preparation of human albumin in a single dose container for intravenous administration. Each 10mL, 20mL, 50mL & 100 mL contains 2g, 4g, 10g and 20g of albumin and is prepared from qualified human plasma. The process includes several chromatographic steps and viral inactivation steps. The manufacturing process uses plasma collected from the donors who are screened for their history as per guidelines laid down by the regulatory authorities. Their blood is screened for the mandatory infectious diseases. Only on being declared negative to HbsAg, HCV and HIV antibodies the plasma is used for processing..
CLINICAL PHARMACOLOGY
Albumin is a highly soluble, globular protein (molecular weight 66,500), accounting for 70-80% of the colloid osmotic pressure of plasma. Therefore, albumin is important in regulating the osmotic pressure of plasma. Human Normal Albumin IP 20% solution will increase the circulating plasma volume. This extra fluid reduces haemoconcentration and decreases blood viscosity. The degree and duration of volume expansion depends upon the initial blood volume. With patients treated for
diminished blood volume, the effect of infused albumin may persist for many hours; however, in patients with normal volume, the duration will be shorter
Presentation
Human Normal Albumin IP 20% available as intravenous infusion in 2g/ 10mL, 4g/ 20mL, 10g/ 50mL and 20g/ 100 mL USP
Type I Glass Vial.
Storage
Store the container between 2OC to 25OC. Do not freeze. Protect from light.
Keep out of reach of children.
Shelf Life: 36 months
Special Precaution for Disposal and Handling
Albumin solutions must not be diluted with water for injection as this may cause haemolysis in recipients. Any unused
medicinal product or waste material should be disposed-off in accordance with local regulatory requirements.